Research
The immune system exists in an intricate balance. Inappropriate response to the body’s own antigens leads to autoimmunity. In contrast, lack of appropriate response to foreign invaders results in immunodeficiency. The thymus plays a key role in establishing this balance by presenting the body’s self-antigens to developing T cells in a process called central tolerance induction.
The Meyer lab studies how the organisation of the thymus and the cells therein contribute to successful T cell development leading to a competent immune system with adequate effector function.
Regulation of promiscuous gene expression
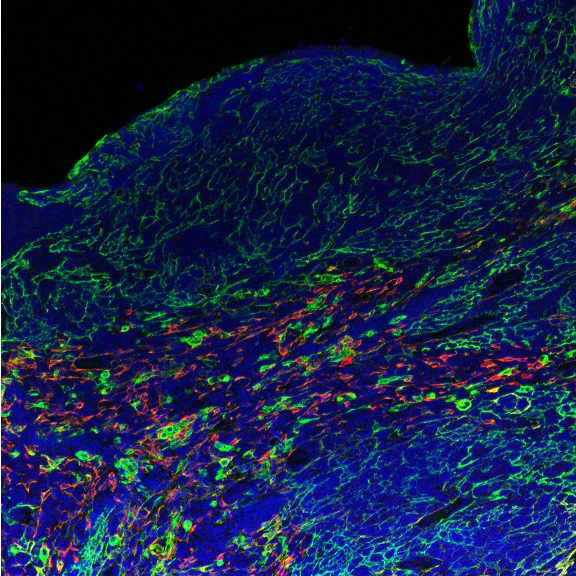
Within the thymus, T cell progenitors known as thymocytes interact with thymic epithelial cells to select for T cells with functional T cell receptors and against T cells that bind strongly to self-antigens. The latter, negative selection process that induces central tolerance largely depends on antigen presentation in the thymic medulla. Medullary thymic epithelial cells express nearly all peripheral antigens, representing essentially all tissues of the body. T cells that react to these peripheral self-antigens are removed to ensure that no autoimmune responses occur. There are many open questions regarding this extraordinary process, from the molecular factors responsible for this promiscuous gene expression, to the tissue-level regulation of the thymic epithelial cells driving it. Many of these questions hinge on the impact of regulation on the thymic selection process.
We study the regulation of thymic gene expression in human and mouse thymus, both on molecular and tissue-wide level to understand the diversity and structure of the antigen space available for T cell education.
Genetic and epigenetic drivers of thymocyte development and differentiation
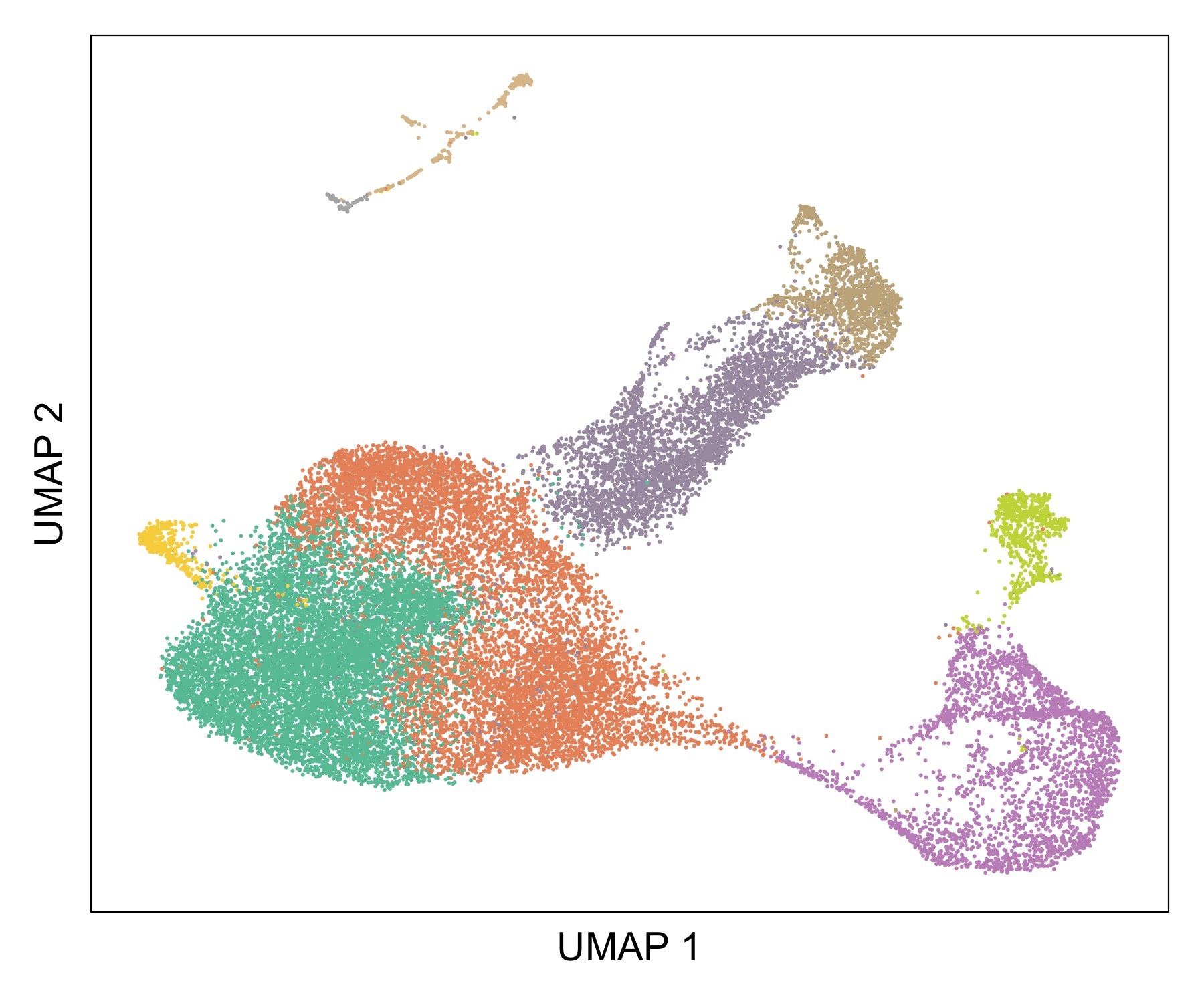
In the thymus, T cell progenitors give rise to a variety of T cell lineages, an intricate process tightly regulated by the combination of environmental signals, transcription factors as well as genetic and epigenetic factors.
We combine experimental and high-throughput sequencing approaches to study the role of genetic and epigenetic factors in regulating thymocytes development and differentiation.
Comprehensive model of central tolerance induction
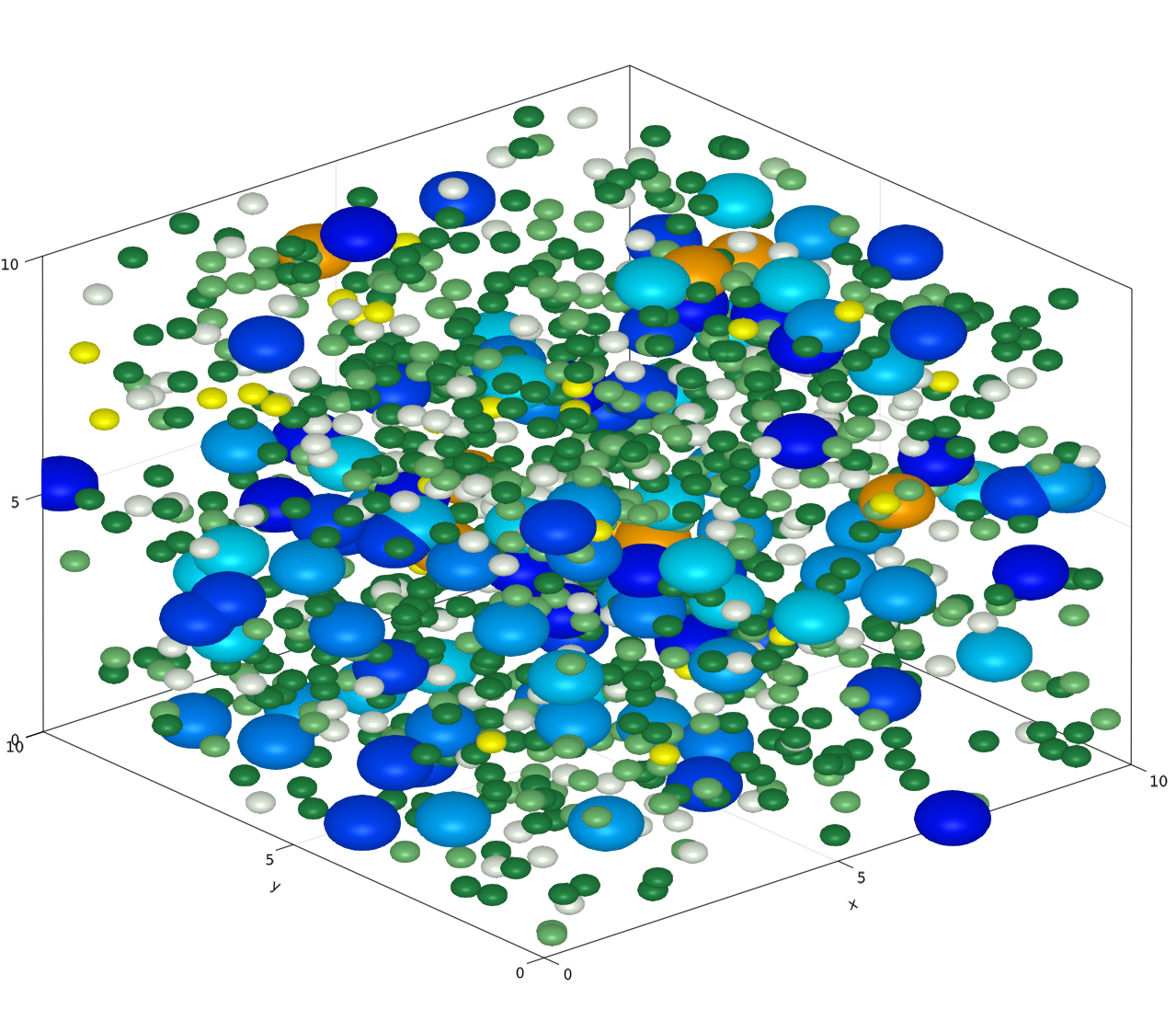
Thymus biology has been well characterized in qualitative terms: the developmental stages of thymocytes and thymic epithelium based on the expression of surface markers are well-known, and key molecular players like Aire and Fezf2 have been identified. In addition, quantitative aspects of the development process have been described: thymocytes spend about 4-5 days in the thymic medulla making 500-2000 contacts with antigen-presenting cells, and the percentage of antigens expressed in the mTEC population reaches about 85%. Despite these major achievements, a comprehensive understanding of how these aspects and thymic gene regulation integrate to achieve immune balance is missing. To efficiently explore the thymic education process, we are developing an in silico model of the thymus. This computational system allows us to explicitly model the interactions between T cells and thymic epithelial cells and gain insight into how an effective, yet self-tolerant, immune system is developed.